Published on July 11, 2022
Our motivation: Joplin, Missouri is part of the Tri-State Mining District that operated lead and zinc mines from 1850 to 1950.1 Historic pit mining resulted in large ore waste piles, groundwater-filled cavities, and heavily polluted soil and waters. Water resources in Joplin, MO are within the Orongo-Duenweg Superfund site, one of the nation’s largest EPA Superfund-designated areas. Small streams are considered low priority bodies of water for remediation and still remain impaired. Not only are the high levels of metal contamination a risk to public health and safety but also to ecosystem health and function. This project focuses on urban stream Lone Elm Reach, Joplin, MO, which suffers from heavy metal pollution (Figure 1).

The intent of this research is to shed new light on a historic topic by analyzing how the quality and quantity of dissolved organic carbon (DOC) changes along the Lone Elm Stream. Dissolved organic carbon is an important nutrient source for aquatic organisms.2 Recent laboratory studies have indicated that iron can react with DOC, making it very difficult to break down by microorganisms; However, limited studies detail where this reaction may occur in real systems.3
Changes to the chemical structure and size of DOC are also important in understanding the role of carbon in the bioavailability of dissolved metals to microorganisms.2 One critical stream function is microbial respiration, and it is dependent on the quality and quantity of DOC. Therefore, surveying DOC composition and concentration can provide more insight into the interactions and impacts that DOC and heavy metals have in impaired systems. An understanding of the environmental factors that drive DOC reactions with metals can lead to better managed resources, restoration of ecosystem functions, and mitigation of metal transport in surface waters.4
Fieldwork and methods: For the team’s first field survey, samples were collected on February 28, 2022. Three sections of the stream were evaluated: upstream of the mine adit, the mine adit itself, and downstream of the mine adit. The mine adit is where groundwater steadily flows through a small drainage creek before joining the main Lone Elm stream. From each section, four water samples and two sediment samples were taken. Sampling techniques included using acid and base washed containers and rinsing the tools in the field. Extra caution was taken when sampling the mine adit drainage creek to protect research safety (Figure 2). The deionized (DI) water sample was brought to the site and handled like all other samples to serve as a field blank. Water and sediment samples were stored on ice. A calibrated YSI EXO1 probe was utilized for dissolved oxygen (%), electrical conductivity (µS/cm), temperature (°C), and turbidity (NTU) measurements in the field. pH was measured via an Orion Star Thermo Fischer 410 pH meter in the lab.

In the laboratory, raw water was vacuum-filtered with combusted 0.7 µm glass fiber filters. Particulate matter collected on filters were saved and frozen for later analysis of carbon complexed to metals. DOC concentration, or total organic carbon (TOC), tests were measured via a Shimadzu machine. Optical spectroscopy methods will be used after a solid phase extraction is performed to remove metal ion interference. Once the samples are re-suspended in a solution, absorbance and fluorescence testing will begin.
Information about the composition of DOC will be determined from UV-absorption spectral methods.2 Ultraviolet and visible absorbance spectrums (220-800 nm) can be used to relate changes in absorbance increases to DOC quality. Tests like specific UV absorbance at 254 nm (SUVA254) are normalized for DOC concentration and indicate aromatic material in the sample. Aromatic carbon, or DOC with a higher SUVA254, correlates with carbon that is hard to break down, or refractory carbon.5 Such carbon is also more challenging to treat in drinking and wastewater systems.
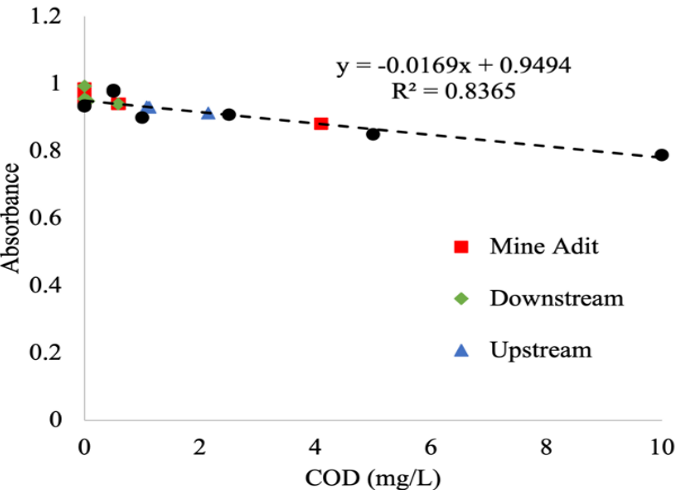
Chemical Oxygen Demand (COD) tests were conducted to assess total oxidizable or oxygen-consuming carbon. Samples were pipetted into Hach Ultra Low Range COD vials and potassium phlalate standards targeting 0-10 ppm (Figure 3). These tests were completed following ATSM 500.8. The COD calibration curve had an R2 of 0.8365 (Figure 3). These tests did not show significant differences across sites, but this particular test is not sensitive to biologically decomposable organic matter. Lastly, trace metals, iron, and zinc in the stream are being determined following EPA Method 200.8. Raw water samples were filtered using a 0.45 um polymer filter for trace metal analysis. Next, samples were acidified with nitric acid to ensure the trace metals stayed dissolved in the samples. To measure the concentrations of the metals, the acidified samples are currently out for analysis at the University of Missouri Research Reactor via inductively coupled plasma mass spectrometry (ICP-MS).
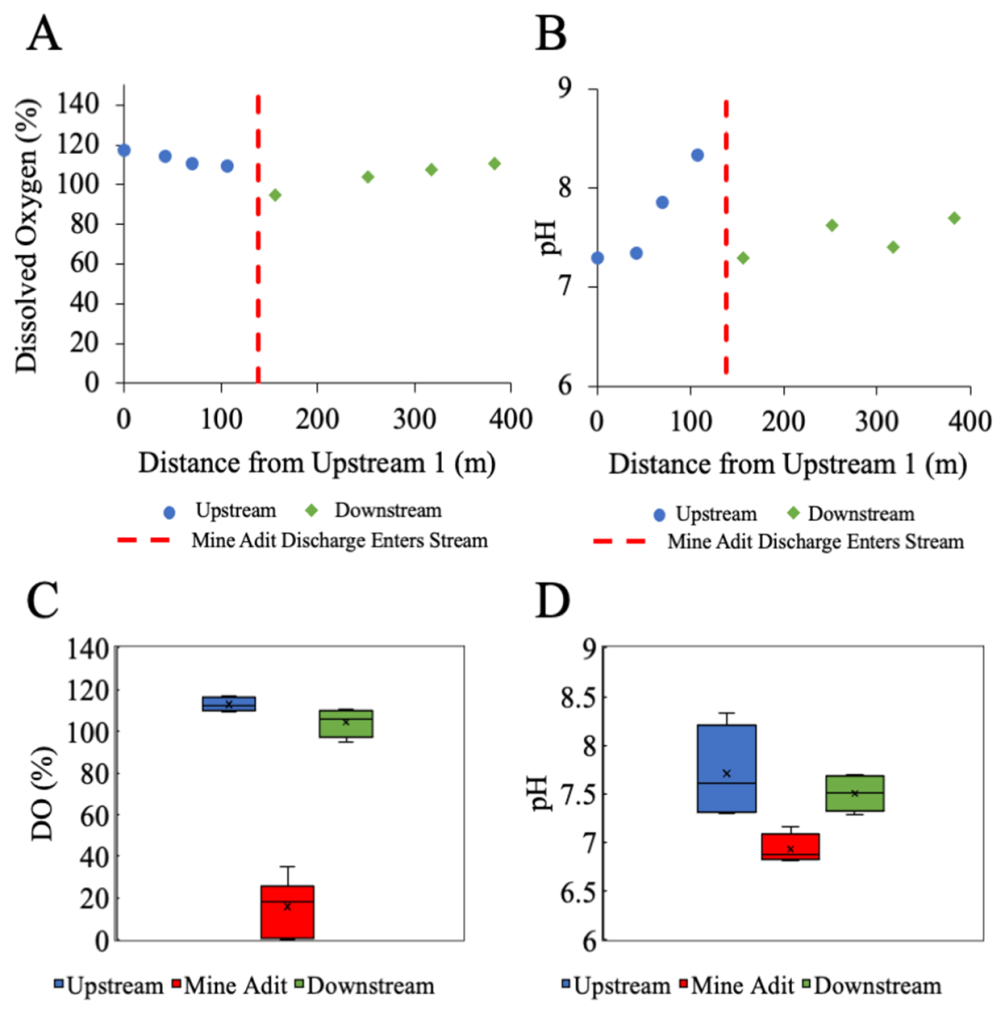
Results and next steps: Our results indicated that the average total organic carbon (TOC) concentrations were 1.47 ± 0.094 mg/L upstream, 1.35 ± 0.11 mg/L downstream, and 1.45 ± 0.25 mg/L at the mine adit. The TOC of the mine adit discharge was not significantly different from the stream by a one-way analysis of variance, ANOVA, test. Figures 4.A and 4.B illustrate dissolved oxygen (DO) and pH as a function of distance from the first upstream site and where the mine adit water discharges into the rest of the stream. Figures 4.C and 4.D display that the mine discharged water that was extremely low in dissolved oxygen = 15.7 ± 12.3 % and pH = 6.93 ± 0.14, and higher in electrical conductivity = 1234.67 ± 8.50 µS/cm. It is clear by Figure 4.A that with the introduction of the low DO mine adit discharge, the DO in the stream drops off and then slightly recovers. In Figure 4.B, the pH is also affected by the mine adit discharge as shown by the drop in pH values after the discharge enters the stream.
With the data that has been analyzed at this point, it is clear that the stream chemistry is being altered by the mine adit discharge. Additional testing will provide our team with better insight into the type of carbon being formed in the stream and the concentration of the metals like Fe, Zn, Br, and Pd, to further uncover the ways in which the stream is being affected by DOC changes. The next steps for this project are to complete the solid phase extraction and ICP-MS to provide such results. A long-term biological oxygen demand (BOD) and microbial respiration incubations via specific oxygen uptake rate (SOUR) will also be optimized for these water samples. BOD and SOUR data will likely be more helpful than COD data because this research focuses on how the metals affect microbial respiration.
In summary, the objective of DOC analysis is to determine the type of the carbon that is formed in Lone Elm – hard or easy to degrade carbon – and if it changes along the stream. Additionally, this research team is interested in discovering if there is a relationship between the type of carbon formed and the concentration of metals. Understanding the interaction between DOC and heavy metals will help researchers explain why microbial respiration is suppressed in this stream. Overall, the research team hopes to develop a mechanistic understanding of stream function drivers to improve stream health assessments. This approach can provide management recommendations for both surface and source waters impacted by chronic pollution across the state of Missouri.
Acknowledgements
Elli Castonguay was supported to do this research by the College of Engineering Fellowship and Civil and Environmental Engineering Department (via PFFFD funding). Dr. Sarah Fischer, Civil and Environmental Engineering, served as Elli’s research mentor and assisted in editing this article. We greatly thank Assistant Professor Alba Argerich, School of Natural Resources, who mentored and supported fieldwork, reviewed materials and shared of field equipment. Ph.D. Candidate Jessi Wilson also assisted with fieldwork logistics and significant background information on the project. Angel Colon conducted total organic carbon analysis for this project via a Shimadzu machine.
About the Author

Elli is going into her senior year as an Honors Civil and Environmental Engineering Student at the University of Missouri in Columbia, MO. Elli serves as the current treasurer for the Water and Environmental Technologists club as well as former president for the Mizzou Club Gymnastics team. Being an undergrad researcher has been an amazing opportunity for Elli to gain exposure to higher levels of learning and hands-on experiences. Additionally, Elli was able to present this research in the student poster competition at the joint annual meeting for American Water Works Association and Missouri Water Environment Association (AWWA/MWEA) on March 28, 2022 (Figure 5). Elli is excited to continue participation in this research and solve similar water related issues in her future career. Overall, Elli’s main goal is to help find solutions to climate change, resource scarcity and sustainability-related issues in her community and beyond.